The Electron
Joseph J. Thomson
The experiments discussed in this paper were undertaken in the hope of gaining some information as to the nature of the Cathode Rays. The most diverse opinions are held as to these rays; according to the almost unanimous opinion of German physicists they are due to some process in the aether to which inasmuch as in a uniform magnetic field their course is circular and not rectilinear—no phenomenon hitherto observed is analogous: another view of these rays is that, so far from being wholly aetherial, they are in fact wholly material, and that they mark the paths of particles of matter charged with negative electricity. It would seem at first sight that it ought not to be difficult to discriminate between views so different, yet experience shows that this is not the case, as amongst the physicists who have most deeply studied the subject can be found supporters of either theory.
The electrified-particle theory has for purposes of research a great advantage over the aetherial theory, since it is definite and its consequences can be predicted; with the aetherial theory it is impossible to predict what will happen under any given circumstances, as on this theory we are dealing with hitherto unobserved phenomena in the aether, of whose laws we are ignorant.
The following experiments were made to test some of the consequences of the electrified-particle theory.
Charge Carried by the Cathode Rays.
If these rays are negatively electrified particles, then when they enter an enclosure they ought to carry into it a charge of negative electricity. This has been proved to be the case by Perrin, who placed in front of a plane cathode two coaxial metallic cylinders which were insulated from each other: the outer of these cylinders was connected with the earth, the inner with a gold-leaf electroscope. These cylinders were closed except for two small holes, one in each cylinder, placed so that the cathode rays could pass through them into the inside of the inner cylinder. Perrin found that when the rays passed into the inner cylinder the electroscope received a charge of negative electricity, while no charge went to the electroscope when the rays were deflected by a magnet so as no longer to pass through the hole.
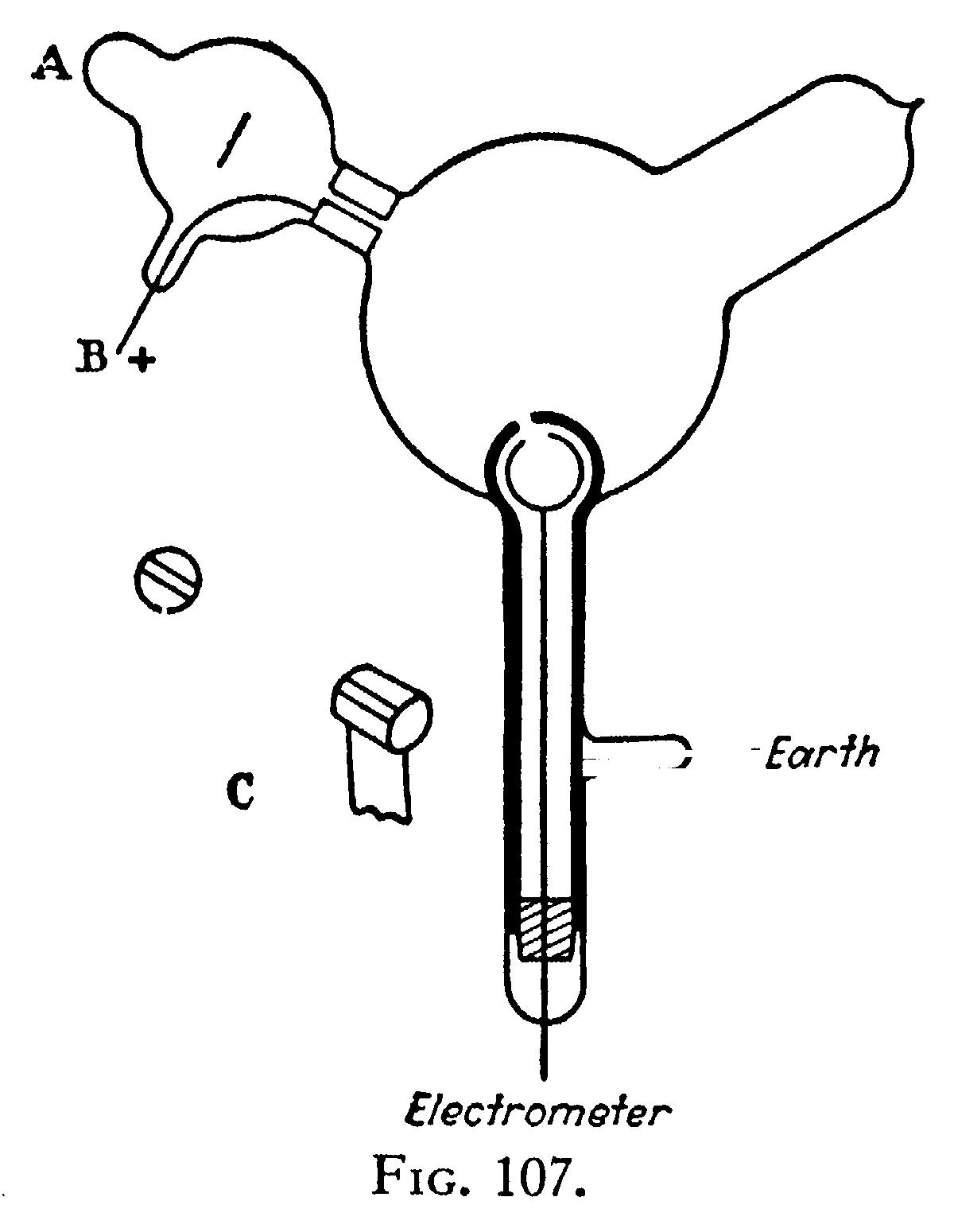
This experiment proves that something charged with negative electricity is shot off from the cathode, travelling at right angles to it, and that this something is deflected by a magnet; it is open, however, to the objection that it does not prove that the cause of the electrification in the electroscope has anything to do with the cathode rays. Now the supporters of the aetherial theory do not deny that electrified particles are shot off from the cathode; they deny, however, that these charged particles have any more to do with the cathode rays than a rifle-bail has with the flash when a rifle is fired. I have therefore repeated Perrin’s experiment in a form which is not open to this objection. The arrangement used was as follows:—Two coaxial cylinders (Fig. 107) with silts in them are placed in a bulb connected with the discharge-tube; the cathode rays from the cathode A pass into the bulb through a slit in a metal plug fitted into the neck of the tube; this plug is connected with the anode and is put to earth. The cathode rays thus do not fail upon the cylinders unless they are deflected by a magnet. The outer cylinder is connected with the earth, the inner with the electrometer. When the cathode rays (whose path was traced by the phosphorescence on the glass) did not fall on the slit, the electrical charge sent to the electrometer when the induction-coil producing the rays was set in action was small and irregular; when, however, the rays were bent by a magnet so as to fall on the slit there was a large charge of negative electricity sent to the electrometer. I was surprised at the magnitude of the charge; on some occasions enough negative electricity went through the narrow slit into the inner cylinder in one second to alter the potential of a capacity of 1.5 microfarads by 20 volts. If the rays were so much bent by the magnet that they overshot the silts in the cylinder, the charge passing into the cylinder fell again to a very small fraction of its value when the aim was true. Thus this experiment shows that however we twist and deflect the cathode rays by magnetic forces, the negative electrification follows the same path as the rays, and that this negative electrification is indissolubly connected with the cathode rays.
When the rays are turned by the magnet so as to pass through the slit into the inner cylinder, the deflexion of the electrometer connected with this cylinder increases up to a certain value, and then remains stationary although the rays continue to pour into the cylinder. This is due to the fact that the gas in the bulb becomes a conductor of electricity when the cathode rays pass through it, and thus, though the inner cylinder is perfectly insulated when the rays are not passing, yet as soon as the rays pass through the bulb the air between the inner cylinder and the outer one becomes a conductor, and the electricity escapes from the inner cylinder to the earth. Thus the charge within the inner cylinder does not go on continually increasing; the cylinder settles down into a state of equilibrium in which the rate at which it gains negative electricity from the rays is equal to the rate at which it loses it by conduction through the air. If the inner cylinder has initially a positive charge it rapidly loses that charge and acquires a negative one; while if the initial charge is a negative one, the cylinder will leak if the initial negative potential is numerically greater than the equilibrium value.
Deflexion of the Cathode Rays by an Electrostatic Field.
An objection very generally urged against the view that the cathode rays are negatively electrified particles, is that hitherto no deflexion of the rays has been observed under a small electrostatic force, and though the rays are deflected when they pass near electrodes connected with sources of large differences of potential, such as induction-coils or electrical machines, the deflexion in this case is regarded by the supporters of the aetherial theory as due to the discharge passing between the electrodes, and not primarily to the electrostatic field. Hertz made the rays travel between two parallel plates of metal placed inside the discharge-tube, but found that they were not deflected when the plates were connected with a battery of storage-cells; on repeating this experiment I at first got the same result, but subsequent experiments showed that the absence of deflexion is due to the conductivity conferred on the rarefied gas by the cathode rays. On measuring this conductivity it was found that it diminished very rapidly as the exhaustion increased; it seemed then that on trying Hertz’s experiment at very high exhaustions there might be a chance of detecting the deflexion of the cathode rays by an electrostatic force.
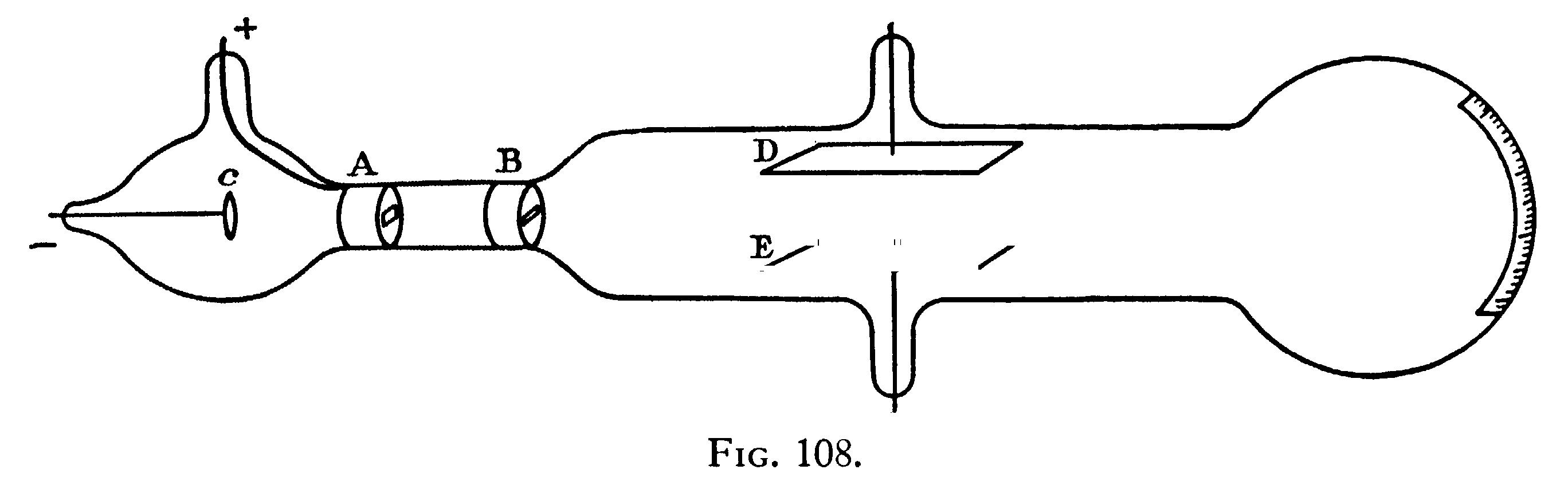
The apparatus used is represented in Fig. 108.
The rays from the cathode C pass through a slit in the anode A, which is a metal plug fitting tightly into the tube and connected with the earth; after passing through a second slit in another earth-connected metal plug B, they travel between two parallel aluminum plates about 5 cm. long and 2 broad and at a distance of 1.5 cm. apart; they then fall on the end of the tube and produce a narrow well-defined phosphorescent patch. A scale pasted on the outside of the tube serves to measure the deflexion of this patch. At high exhaustions the rays were deflected when the two aluminum plates were connected with the terminals of a battery of small storage-cells; the rays were depressed when the upper plate was connected with the negative pole of the battery, the lower with the positive, and raised when the upper plate was connected with the positive, the lower with the negative pole. The deflexion was proportional to the difference of potential between the plates, and I could detect the deflexion when the potential-difference was as small as two volts. It was only when the vacuum was a good one that the deflexion took place, but that the absence of deflexion is due to the conductivity of the medium is shown by what takes place when the vacuum has just arrived at the stage at which the deflexion begins. At this stage there is a deflexion of the rays when the plates are first connected with the terminals of the battery, but if this connexion is maintained the patch of phosphorescence gradually creeps back to its undeflected position. This is just what would happen if the space between the plates were a conductor, though a very bad one, for then the positive and negative ions between the plates would slowly diffuse, until the positive plate became coated with negative ions, the negative plate with positive ones; thus the electric intensity between the plates would vanish and the cathode rays be free from electrostatic force. Another illustration of this is afforded by what happens when the pressure is low enough to show the deflexion and a large difference of potential, say 200 volts, is established between the plates; under these circumstances there is a large deflexion of the cathode rays, but the medium under the large electromotive force breaks down every now and then and a bright discharge passes between the plates; when this occurs the phosphorescent patch produced by the cathode rays jumps back to its undeflected position. When the cathode rays are deflected by the electrostatic field, the phosphorescent band breaks up into several bright bands separated by comparatively dark spaces; the phenomena are exactly analogous to those observed by Birke-land when the cathode rays are deflected by a magnet, and called by him the magnetic spectrum.
A series of measurements of the deflexion of the rays by the electrostatic force under various circumstances will be found later on in the part of the paper which deals with the velocity of the rays and the ratio of the mass of the electrified particles to the charge carried by them. It may, however, be mentioned here that the deflexion gets smaller as the pressure diminishes, and when in consequence the potential-difference in the tube in the neighborhood of the cathode increases.
Magnetic Deflexion of the Cathode Rays in Different Gases.
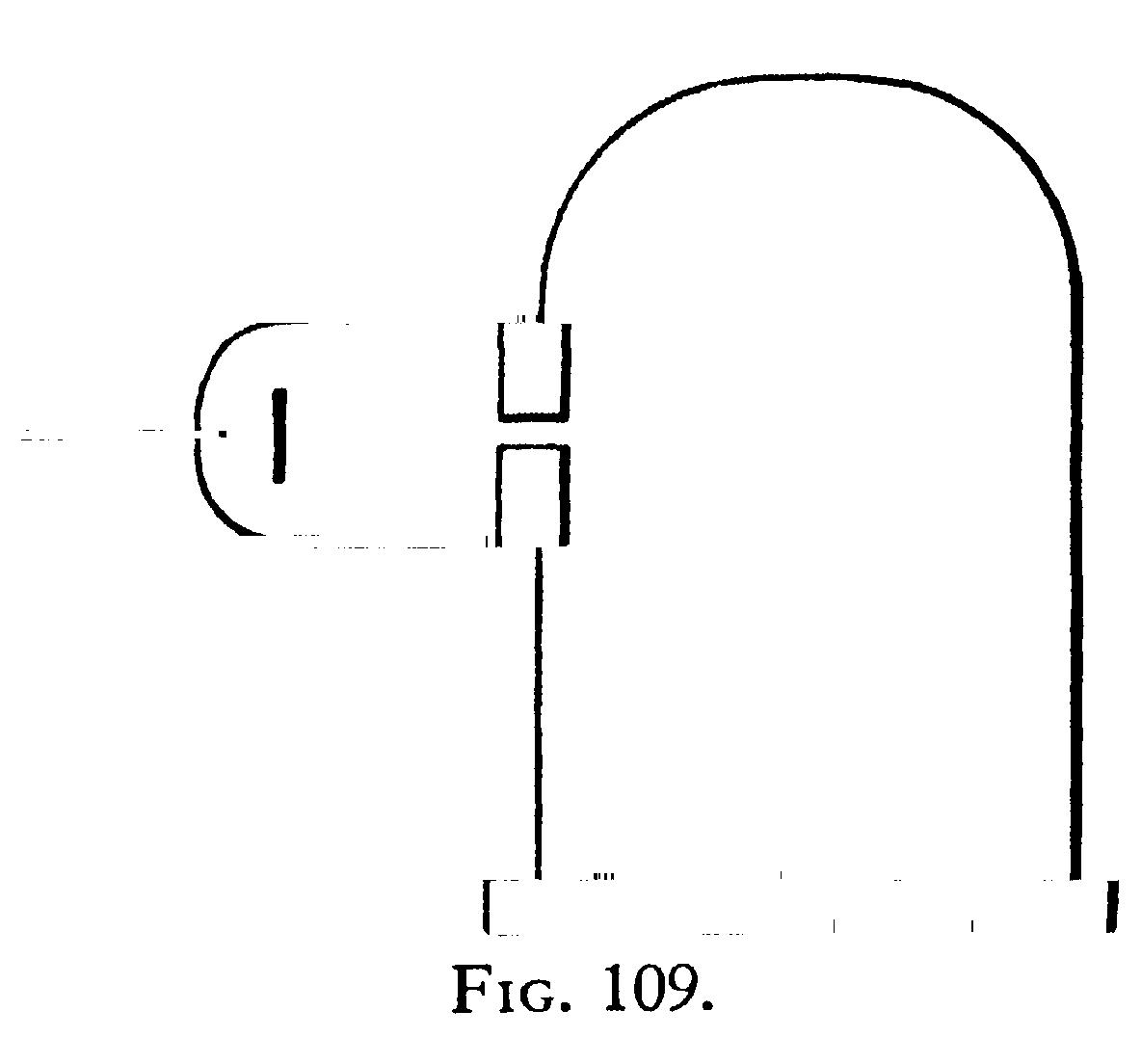
The deflexion of the cathode rays by the magnetic field was studied with the aid of the apparatus shown in Fig. 109. The cathode was placed in a side-tube fastened on to a bell-jar; the opening between this tube and the bell-jar was closed by a metallic plug with a slit in it; this plug was connected with the earth, and was used as the anode. The cathode rays passed through the silt in this plug into the bell-jar, passing in front of a vertical plate of glass ruled into small squares. The bell-jar was placed between two large parallel coils arranged as a Helmholtz galvanometer. The course of the rays was determined by taking photographs of the bell-jar when the cathode rays were passing through it; the divisions on the plate enabled the path of the rays to be determined. Under the action of the magnetic field the narrow beam of cathode rays spreads out into a broad fan-shaped luminosity in the gas. The luminosity in this fan is not uniformly distributed, but is condensed along certain lines. The phosphorescence on the glass is also not uniformly distributed; it is much spread out, showing that the beam consists of rays which are not all deflected to the same extent by the magnet. The luminosity on the glass is crossed by bands along which the luminosity is very much greater than in the adjacent parts. These bright and dark bands are called by Birkeland, who first observed them, the magnetic spectrum. The brightest spots on the glass are by no means always the terminations of the brightest streaks of luminosity in the gas; in fact, in some cases a very bright spot on the glass is not connected with the cathode by any appreciable luminosity, though there may be plenty of luminosity in other parts of the gas. One very interesting point brought out by the photographs is that in a given magnetic field, and with a given mean potential-difference between the terminals, the path of the rays is independent of the nature of the gas. Photographs were taken of the discharge in hydrogen, air, carbonic acid, methyl iodide, i.e., in gases whose densities range from 1 to 70, and yet, not only were the paths of the most deflected rays the same in all cases, but even the details, such as the distribution of the bright and dark spaces, were the same; in fact, the photographs could hardly be distinguished from each other. It is to be noted that the pressures were not the same; the pressures in the different gases were adjusted so that the mean potential-differences between the cathode and the anode were the same in all the gases. When the pressure of a gas is lowered, the potential-difference between the terminals increases, and the deflexion of the rays produced by a magnet diminishes, or at any rate the deflexion of the rays when the phosphorescence is a maximum diminishes. If an air-break is inserted an effect of the same kind is produced.
In the experiments with different gases, the pressures were as high as was consistent with the appearance of the phosphorescence on the glass, so as to ensure having as much as possible of the gas under consideration in the tube.
As the cathode rays carry a charge of negative electricity, are deflected by an electrostatic force as if they were negatively electrified, and are acted on by a magnetic force in just the way in which this force would act on a negatively electrified body moving along the path of these rays, I can see no escape from the conclusion that they are charges of negative electricity carried by particles of matter. The question next arises, What are these particles? are they atoms, or molecules, or matter in a still finer state of subdivision? To throw some light on this point, I have made a series of measurements of the ratio of the mass of these particles to the charge carried by it. To determine this quantity, I have used two independent methods. The first of these is as follows:—Suppose we consider a bundle of homogeneous cathode rays. Let m be the mass of each of the particles, e the charge carried by it. Let N be the number of particles passing across any section of the beam in a given time; then Q the quantity of electricity carried by these particles is given by the equation
We can measure Q if we receive the cathode rays in the inside of a vessel connected with an electrometer. When these rays strike against a solid body, the temperature of the body is raised; the kinetic energy of the moving particles being converted into heat; if we suppose that all this energy is converted into heat, then if we measure the increase in the temperature of a body of known thermal capacity caused by the impact of these rays, we can determine W, the kinetic energy of the particles, and if v is the velocity of the particles,
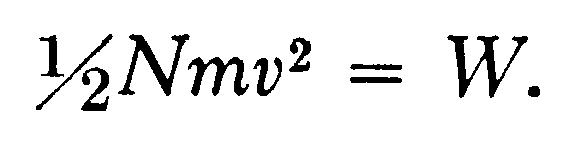
If p is the radius of curvature of the path of these rays in a uniform magnetic field H, then
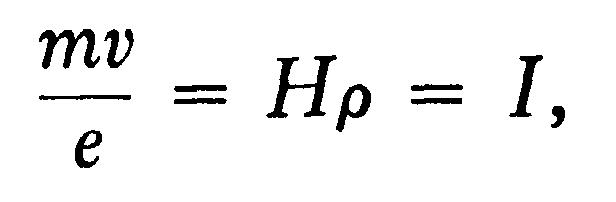
where I is written for Hp for the sake of brevity. From these equations we get
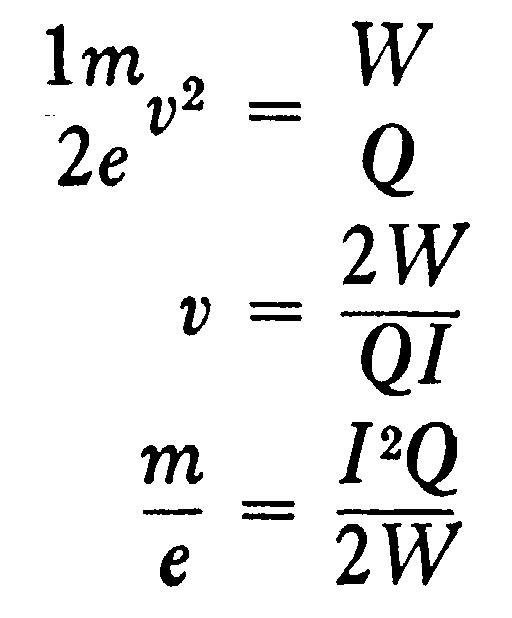
Thus, if we know the values of Q, W, and I, we can deduce the values of v and m/e.
To measure these quantities, I have used tubes of three different types. The first I tried is like that represented in Fig. 108, except that the plates E and D are absent, and two coaxial cylinders are fastened to the end of the tube. The rays from the cathode C fail on the metal plug B, which is connected with the earth, and serves for the anode; a horizontal slit is cut in this plug. The cathode rays pass through this silt, and then strike against the two coaxial cylinders at the end of the tube; slits are cut in these cylinders, so that the cathode rays pass into the inside of the inner cylinder. The outer cylinder is connected with the earth, the inner cylinder, which is insulated from the outer one, is connected with an electrometer, the deflexion of which measures Q, the quantity of electricity brought into the inner cylinder by the rays. A thermo-electric couple is placed behind the slit in the inner cylinder; this couple is made of very thin strips of iron and copper fastened to very fine iron and copper wires. These wires passed through the cylinders, being insulated from them, and through the glass to the outside of the tube, where they were connected with a low-resistance galvanometer, the deflexion of which gave data for calculating the rise of temperature of the junction produced by the impact against it of the cathode rays. The strips of iron and copper were large enough to ensure that every cathode ray which entered the inner cylinder struck against the junction. In some of the tubes the strips of iron and copper were placed end to end, so that some of the rays struck against the iron, and others against the copper; in others, the strip of one metal was placed in front of the other; no difference, however, could be detected between the results got with these two arrangements. The strips of iron and copper were weighed, and the thermal capacity of the junction calculated. In one set of junctions this capacity was 5 x 10–3, in another 3 X 10–3. If we assume that the cathode rays which strike against the junction give their energy up to it the deflexion of the galvanometer gives us W or 1/2Nmv2.
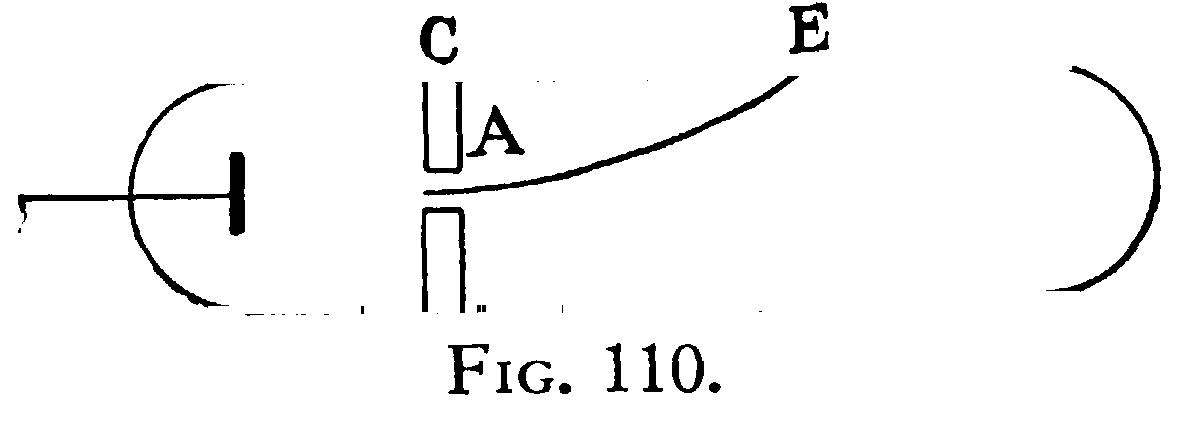
The value of I, i.e., Hp, where p is the curvature of the path of the rays in a magnetic field of strength H was found as follows: The tube was fixed between two large circular coils placed parallel to each other, and separated by a distance equal to the radius of either; these coils produce a uniform magnetic field, the strength of which is got by measuring with an ammeter the strength of the current passing through them. The cathode rays are thus in a uniform field, so that their path is circular. Suppose that the rays, when deflected by a magnet, strike against the glass of the tube at E (Fig. 110), then, if p is the radius of the circular path of the rays, thus, if we measure CE and AC we have the means of determining the radius of curvature of the path of the rays.
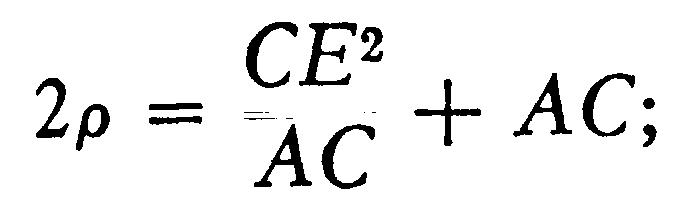
The determination of p is rendered to some extent uncertain, in consequence of the pencil of rays spreading out under the action of the magnetic field, so that the phosphorescent patch of E is several millimetres long; thus values of p differing appreciably from each other will be got by taking E at different points of this phosphorescent patch. Part of this patch was, however, generally considerably brighter than the rest; when this was the case, E was taken as the brightest point; when such a point of maximum brightness did not exist, the middle of the patch was taken for E. The uncertainty in the value of p thus introduced amounted sometimes to about 20 percent; by this I mean that if we took E first at one extremity of the patch and then at the other, we should get values of p differing by this amount.
The measurement of Q, the quantity of electricity which enters the inner cylinder, is complicated by the cathode rays making the gas through which they pass a conductor, so that though the insulation of the inner cylinder was perfect when the rays were off, it was not so when they were passing through the space between the cylinders; this caused some of the charge communicated to the inner cylinder to leak away so that the actual charge given to the cylinder by the cathode rays was larger than that indicated by the electrometer.
To make the error from this cause as small as possible, the inner cylinder was connected to the largest capacity available, 1.5 micro-farad, and the rays were only kept on for a short time, about 1 or 2 seconds, so that the alteration in potential of the inner cylinder was not large, ranging in the various experiments from about .5 to 5 volts. Another reason why it is necessary to limit the duration of the rays to as short a time as possible, is to avoid the correction for the loss of heat from the thermo-electric junction by conduction along the wires; the rise in temperature of the junction was of the order 2°C.; a series of experiments showed that with the same tube and the same gaseous pressure Q and W were proportional to each other when the rays were not kept on too long.
Tubes of this kind gave satisfactory results, the chief drawback being that sometimes in consequence of the charging up of the glass of the tube, a secondary discharge started from the cylinder to the walls of the tube, and the cylinders were surrounded by glow; when this glow appeared, the readings were very irregular; the glow could, however, be got rid of by pumping and letting the tube rest for some time. The results got with this tube are given in the Table under the heading Tube 1.
The second type of tube was like that used for photographing the path of the rays (Fig. 109); double cylinders with a thermoelectric junction like those used in the previous tube were placed in the line of fire of the rays, the inside of the bell-jar was lined with copper gauze connected with the earth. This tube gave very satisfactory results; we were never troubled with any glow round the cylinders, and the readings were most concordant; the only drawback was that as some of the connexions had to be made with sealing wax, it was not possible to get the highest exhaustions with this tube, so that the range of pressure for this tube is less than that for Tube 1. The results got with this tube are given in the Table under the heading Tube 2.
The third type of tube was similar to the first, except that the openings in the two cylinders were made very much smaller; in this tube the slits in the cylinders were replaced by small holes, about 1.5 millim. in diameter. In consequence of the smallness of the openings, the magnitude of the effects was very much reduced; in order to get measurable results it was necessary to reduce the capacity of the condenser in connexion with the inner cylinder to .15 microfarad, and to make the galvanometer exceedingly sensitive, as the rise in temperature of the thermo-electric junction was in these experiments only about .5C. on the average. The results obtained in this tube are given in the Table under the heading Tube 3.
The Tables in which the results of the several experiments are presented are omitted.
It will be noticed that the value of m/e is considerably greater for Tube 3, where the opening is a small hole, than for Tubes 1 and 2, where the opening is a silt of much greater area. I am of opinion that the values of m/e got from Tubes 1 and 2 are too small, in consequence of the leakage from the inner cylinder to the outer by the gas being rendered a conductor by the passage of the cathode rays.
It will be seen from these tables that the value of m/e is independent of the nature of the gas. Thus, for the first tube the mean for air is .40 X 10–7, for hydrogen .42 X 10–7, and for carbonic acid gas .4 X 10–7; for the second tube the mean for air is .52 X 10–7, for hydrogen .50 X 10–7, and for carbonic acid gas .54 X 10–7.
Experiments were tried with electrodes made of iron instead of aluminum; this altered the appearance of the discharge and the value of v at the same pressure, the values of m/e were, however, the same in the two tubes; the effect produced by different metals on the appearance of the discharge will be described later on.
In all the preceding experiments, the cathode rays were first deflected from the cylinder by a magnet, and it was then found that there was no deflexion either of the electrometer or the galvanometer, so that the deflexions observed were entirely due to the cathode rays; when the glow mentioned previously surrounded the cylinders there was a deflexion of the electrometer even when the cathode rays were deflected from the cylinder.
Before proceeding to discuss the results of these measurements I shall describe another method of measuring the quantities m/e and v of an entirely different kind from the preceding; this method is based upon the deflexion of the cathode rays in an electrostatic field. If we measure the deflexion experienced by the rays when traversing a given length under a uniform electric intensity, and the deflexion of the rays when they traverse a given distance under a uniform magnetic field, we can find the values of m/e and v in the following way:
Let the space passed over by the rays under a uniform electric intensity F be t, the time taken for the rays to traverse this space is l/v, the velocity in the direction of F is therefore
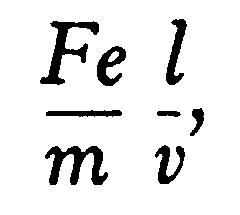
so that qqq, the angle through which the rays are deflected when they leave the electric field and enter a region free from electric force, is given by the equation
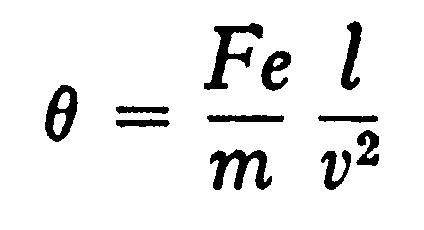
If, instead of the electric intensity, the rays are acted on by a magnetic force H at right angles to the rays, and extending across the distance l, the velocity at right angles to the original path of the rays is
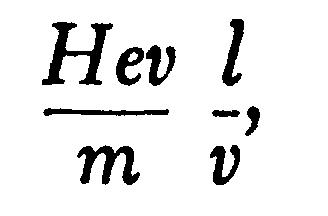
so that qqq, the angle through which the rays are deflected when they leave the magnetic field, is given by the equation
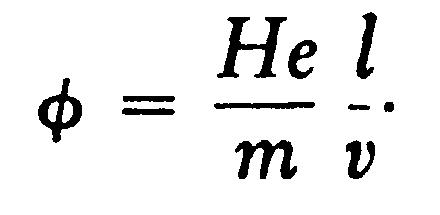
From these equations we get
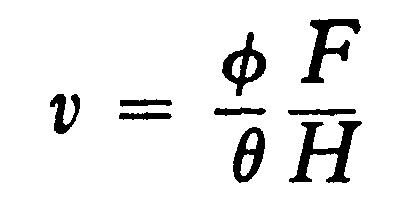
and
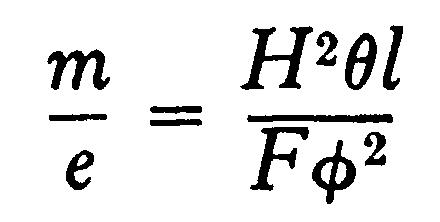
In the actual experiments H was adjusted so that qqq = qqq, in this ease the equations become
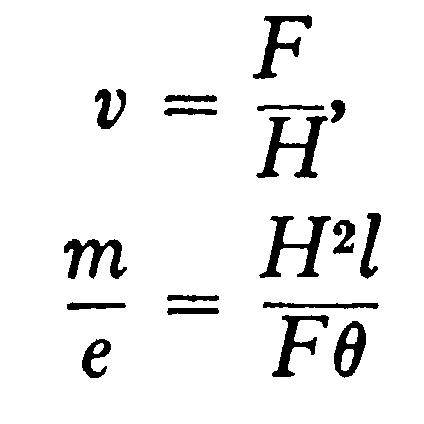
The apparatus used to measure v and m/e by this means is that represented in Fig. 108. The electric field was produced by connecting the two aluminum plates to the terminals of a battery of storage-cells. The phosphorescent patch at the end of the tube was deflected, and the deflexion measured by a scale pasted to the end of the tube. As it was necessary to darken the room to see the phosphorescent patch, a needle coated with luminous paint was placed so that by a screw it could be moved up and down the scale; this needle could be seen when the room was darkened, and it was moved until it coincided with the phosphorescent patch. Thus when light was admitted, the deflexion of the phosphorescent patch could be measured.
The magnetic field was produced by placing outside the tube two coils whose diameter was equal to the length of the plates; the coils were placed so that they covered the space occupied by the plates, the distance between the coils was equal to the radius of either. The mean value of the magnetic force over the length l was determined in the following way: a narrow coil C whose length was l, connected with a ballistic galvanometer, was placed between the coils; the plane of the windings of C was parallel to the planes of the coils; the cross section of the coil was a rectangle 5 cm. by 1 cm. A given current was sent through the outer coils and the kick a of the galvanometer observed when this current was reversed. The coil C was then placed at the centre of two very large coils, so as to be in a field of uniform magnetic force: the current through the large coils was reversed and the kick B of the galvanometer again observed; by comparing a and B we can get the mean value of the magnetic force over a length l; this was found to be
60 × i
where i is the current flowing through the coils.
A series of experiments was made to see if the electrostatic deflexion was proportional to the electric intensity between the plates; this was found to be the case. In the following experiments the current through the coils was adjusted so that the electrostatic deflexion was the same as the magnetic:
| Gas |
qqq |
H |
F |
l |
m/e |
v |
| Air |
8/110 |
5.5 |
1.5 × 1010 |
5 |
1.3 × 10-7 |
2.8 × 10 9 |
| Air |
9.5/110 |
5.4 |
1.5 × 1010 |
5 |
1.1 × 10-7 |
2.8 × 109 |
| Air |
13/110 |
6.6 |
1.5 × 1010 |
5 |
1.2 × 10-7 |
2.3 × 109 |
| Hydrogen |
9/110 |
6.3 |
1.5 × 1010 |
5 |
1.5 × 10-7 |
2.5 × 109 |
| Carbonic acid |
11/110 |
6.9 |
1.5 × 1010 |
5 |
1.5 × 10-7 |
2.2 × 109 |
| Air |
6/110 |
5 |
1.8 × 1010 |
5 |
1.3 × 10-7 |
3.6 × 109 |
| Air |
7/110 |
3.6 |
1 × 1010 |
5 |
1.1 × 10-7 |
2.8 × 109 |
The cathode in the first five experiments was aluminum, m the last two experiments it was made of platinum; in the last experiment Sir William Crookes’s method of getting rid of the mercury vapour by inserting tubes of pounded sulphur, sulphur iodide, and copper filings between the bulb and the pump was adopted. In the calculation of m/e and v no allowance has been made for the magnetic force due to the coil in the region outside the plates; in this region the magnetic force will be in the opposite direction to that between the plates, and will tend to bend the cathode rays in the opposite direction: thus the effective value of H will be smaller than the value used in the equations, so that the values of m/e are larger, and those of v less than they would be if this correction were applied. This method of determining the values of role and v is much less laborious and probably more accurate than the former method; it cannot, however, be used over so wide a range of pressures.
From these determinations we see that the value of m/e is independent of the nature of the gas, and that its value 10–7 is very small compared with the value 10–4, which is the smallest value of this quantity previously known, and which is the value for the hydrogen ion in electrolysis.
Thus for the carriers of the electricity in the cathode rays m/e is very small compared with its value in electrolysis. The small-hess of m/e may be due to the smallness of m or the largeness of e, or to a combination of these two. That the carriers of the charges in the cathode rays are small compared with ordinary molecules is shown, I think, by Lenard’s results as to the rate at which the brightness of the phosphorescence produced by these rays diminishes with the length of path travelled by the ray. If we regard this phosphorescence as due to the impact of the charged particles, the distance through which the rays must travel before the phosphorescence fades to a given fraction (say 1/e, where e = 2.71) of its original intensity, will be some moderate multiple of the mean free path. Now Lenard found that this distance depends solely upon the density of the medium, and not upon its chemical nature or physical state. In air at atmospheric pressure the distance was about half a centimetre, and this must be comparable with the mean free path of the carriers through air at atmospheric pressure. But the mean free path of the molecules of air is a quantity of quite a different order. The carrier, then, must be small compared with ordinary molecules.
The two fundamental points about these carriers seem to me to be (1) that these carriers are the same whatever the gas through which the discharge passes, (2) that the mean free paths depend upon nothing but the density of the medium traversed by these rays.